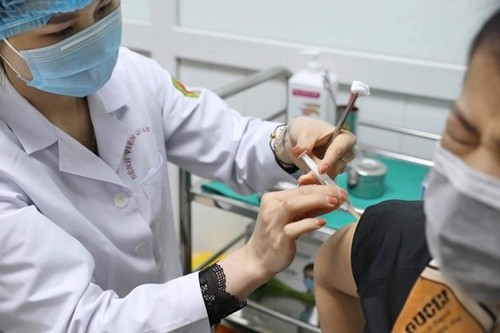
 A volunteer is injected with Nano Covax in phase 2 of human trials. (Photo: hanoimoi.com.vn) A volunteer is injected with Nano Covax in phase 2 of human trials. (Photo: hanoimoi.com.vn) |
They said the 25 microgram dosage proved the most effective and will be used for the third phase of human trials, expected to begin on May 5.
Among the three domestic COVID-19 vaccines being developed, Nano Covax is the fastest-deployed vaccine. Of the other two vaccines, Covivax, has just completed a phase-1 human trial, and a vaccine developed by the Vabiotech Company is scheduled to be tested in humans in June.
Vietnam confirmed 6 new imported COVID-19 cases on Monday evening, all of whom were quarantined upon arrival.
Vietnam has cured 2,516 out of 2,852 COVID-19 patients, 35 died.