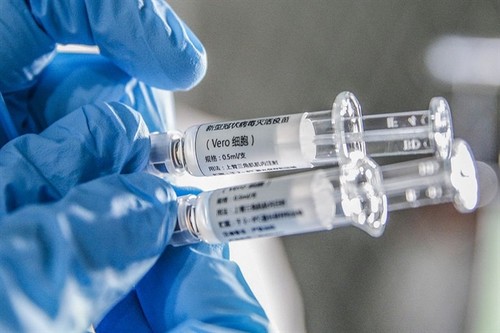
 A staff member displays samples of the COVID-19 inactivated vaccine at Sinovac Biotech Ltd., in Beijing, capital of China. (Photo: XINHUA/VNA) A staff member displays samples of the COVID-19 inactivated vaccine at Sinovac Biotech Ltd., in Beijing, capital of China. (Photo: XINHUA/VNA) |
The National Institute of Hygiene and Epidemiology will be responsible for cooperating with vaccine manufacturers and relevant agencies to ensure the production of this vaccine and its safety and quality.
Sinopharm’s Velo Cell is the third vaccine approved by Vietnam for emergency use, after Sputnik V and AstraZeneca.
The vaccine was approved by China last December and by the WHO for emergency use last month.
Some 42 countries and territories have been using Velo Cell, of which 200 million doses have been shipped worldwide.